Abstract
Introduction: Cytarabine is a backbone of intensive acute myeloid leukemia (AML) chemotherapy; yet it is associated with toxicity which precludes its use in older patients and in those with comorbidities. Aspacytarabine (BST-236) is a prodrug of cytarabine, which is inactive in its intact prodrug form until cytarabine is gradually released in the plasma and inside the cells. Due to the pharmacokinetics and metabolism of aspacytarabine, the peak toxic systemic exposure to cytarabine is decreased, resulting in reduced systemic toxicity with relative sparing of normal tissues, thus enabling delivery of high cytarabine doses to AML patients otherwise unfit to receive intensive cytarabine therapy. Presented herein are the data, including final primary endpoint analysis, from a phase 2b study that recently completed enrollment.
Aims: To evaluate the efficacy and safety of aspacytarabine as a single agent for induction and consolidation therapy of newly diagnosed AML patients unfit for standard induction chemotherapy.
Methods: Aspacytarabine was administered at 6-day courses of 4.5 g/m 2/d (equimolar to 3 g/m 2/d cytarabine) daily 1-hour intravenous infusions, for 1-2 induction and 1-3 consolidation courses, to AML patients age ≥75 years or otherwise unfit for intensive chemotherapy. Patients with secondary or therapy related AML, and patients who received prior hypomethylating agents (HMA) ± venetoclax therapy for a preceding condition were eligible. Primary endpoint is complete remission with complete hematological recovery (CR). Secondary and exploratory endpoints include safety, duration of response (DOR), overall survival (OS), and minimal residual disease (MRD). NCT03435848.
Results: Enrollment into the study was completed in May 2021. Sixty-five newly diagnosed AML patients unfit for standard chemotherapy (median age 75 years, range 54-88 years) were treated with aspacytarabine and completed 1-4 courses (median 1 course) at doses of 4.5 g/m 2/d, including 39 patients (60%) with de novo AML and 26 patients (40%) with secondary AML. Eleven patients (17%) were previously treated for MDS/CMML with HMA (median 14 courses), including 1 patient (2%) with HMA + venetoclax. Thirty-two patients (49%) and 15 patients (23%) had adverse or intermediate European LeukemiaNet (ELN) scores, respectively. The baseline median bone marrow blasts was 43%, and 25 patients (38%) had ECOG PS 2 or 3 at enrollment (Table 1).
Aspacytarabine was well-tolerated in repeated-course administration. Grade ≥3 drug-related adverse events included hematological events and infections, with, importantly, no severe gastrointestinal or neurological events. The 30-day all-cause mortality rate was 12.5% (95% CI, 5.9-23.7).
Of the 65 intention-to-treat AML patients, 24 (37%) achieved a CR following 1 (17 patients) or 2 (7 patients) courses. The median time to complete neutrophil and platelet recovery were 27 days (range 11-39 days) and 26 days (range 18-40), respectively. The CR rates in de novo and secondary AML patients were 44% and 27%, respectively. Twenty-seven percent of the patients with prior HMA therapy achieved a CR, including the 1 patient with prior HMA + venetoclax treatment. The CR rates in patients at the age of ≥75 years and patients with adverse ELN scores were 35% and 34%, respectively.
Of the 21 patients in CR evaluable to date for MRD analysis by multiparameter flow cytometry, 11 (52%) became MRD negative following aspacytarabine treatment.
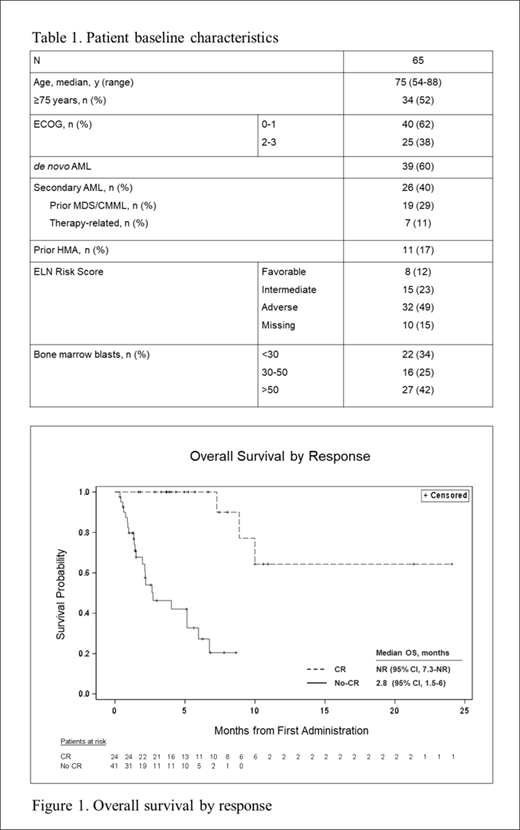
Follow up is ongoing, with a current median duration of follow up of 5.2 months. The median DOR has not yet been reached at 12 months, and median OS of responders not reached at 24 months (Figure 1).
Conclusions: Aspacytarabine appears to be an effective regimen for AML with a considerable reduction of the attendant toxicities that typically are associated with standard intensive cytotoxic therapies. The results of the phase 2b study are consistent with the previously completed phase 1/2a study, which demonstrated safety and a 36% CR rate in a similar population, suggest that aspacytarabine, given as monotherapy, is generally safe and effective as a first-line therapy for AML patients who are unfit for intensive chemotherapy These data support a role for aspacytarabine as a new treatment option for older patients with AML. Furthermore, this agent may serve as a backbone for combination therapy with targeted or other chemotherapy agents, as well as in younger patients with AML.
Altman: Boehringer Ingelheim: Research Funding; BMS: Research Funding; Immunogen: Research Funding; Syros: Consultancy; GlycoMimetics: Other: Participation on an advisory board; Daiichi Sankyo: Consultancy; AbbVie: Consultancy, Other: Advisory Board, Research Funding; Fujifilm: Research Funding; Kura Oncology: Consultancy; Kartos: Research Funding; Biosight: Consultancy, Other: Travel fees, Research Funding; Astellas: Consultancy, Other: Advisory Board, Research Funding; ALZ Oncology: Research Funding; Amgen: Research Funding; Aprea: Research Funding; Theradex: Consultancy, Other: Advisory boards; Kura: Research Funding. Koprivnikar: Bristol Myers Squibb: Speakers Bureau. McCloskey: Amgen: Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Jazz: Consultancy, Speakers Bureau; Pfizer: Consultancy; Novartis: Consultancy; Incyte: Speakers Bureau; BMS: Honoraria, Speakers Bureau; COTA: Other: Equity Ownership. Kota: Pfizer: Consultancy; Novartis: Consultancy; Incyte: Research Funding. Emadi: Secura Bio.: Consultancy; Servier: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; NewLink Genetics: Research Funding; Jazz Pharmaceuticals: Research Funding; KinaRx, Inc.: Membership on an entity's Board of Directors or advisory committees, Other: Co-founder. Zuckerman: BioSight Ltd: Honoraria; AbbVie: Honoraria; Janssen: Honoraria; Orgenesis Inc.: Honoraria; Cellect Biotechnology: Honoraria; Novartis: Honoraria; Gilead Sciences: Honoraria, Speakers Bureau. Levy: Bristol Myers Squibb: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Beigene: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Gilead Sciences, Inc.: Consultancy, Honoraria, Speakers Bureau; Janssen Pharmaceuticals: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Other: Promotional speaker; Amgen Inc.: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; AbbVie: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Karyopharm: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Morphosys: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; Seattle Genetics: Consultancy, Honoraria, Other: Promotional speaker, Speakers Bureau; TG Therapeutics: Consultancy, Honoraria, Speakers Bureau; GSK: Consultancy, Other: Promotional speaker; Epizyme: Consultancy, Other: Promotional speaker; Dova: Consultancy, Other: Promotional speaker. Luger: Syros: Honoraria; Agios: Honoraria; Daiichi Sankyo: Honoraria; Jazz Pharmaceuticals: Honoraria; Brystol Myers Squibb: Honoraria; Acceleron: Honoraria; Astellas: Honoraria; Pfizer: Honoraria; Onconova: Research Funding; Celgene: Research Funding; Biosight: Research Funding; Hoffman LaRoche: Research Funding; Kura: Research Funding. Wolach: Novartis: Consultancy; Amgen: Research Funding; Janssen: Consultancy; Abbvie: Consultancy, Honoraria, Research Funding; Astellas: Consultancy; Neopharm: Consultancy. Percival: AbbVie: Research Funding; Biosight: Research Funding; BMS/Celgene: Research Funding; Cardiff Oncology: Research Funding; Glycomimetics: Research Funding; Nohla therapeutics: Research Funding; Oscotec: Research Funding; Pfizer: Research Funding; Trillium: Research Funding. Roboz: Actinium: Consultancy; Janssen: Consultancy; AstraZeneca: Consultancy; AbbVie: Consultancy; Jazz: Consultancy; MEI Pharma - IDMC Chair: Consultancy; Mesoblast: Consultancy; Glaxo SmithKline: Consultancy; Novartis: Consultancy; Bayer: Consultancy; Amgen: Consultancy; Celgene: Consultancy; Janssen: Research Funding; Daiichi Sankyo: Consultancy; Blueprint Medicines: Consultancy; Bristol Myers Squibb: Consultancy; Helsinn: Consultancy; Jasper Therapeutics: Consultancy; Astellas: Consultancy; Astex: Consultancy; Agios: Consultancy; Pfizer: Consultancy; Otsuka: Consultancy; Roche/Genentech: Consultancy. Levi: AbbVie: Consultancy, Research Funding. Flaishon: BioSight Ltd.: Current Employment. Cohen: BioSight Ltd.: Current Employment. Tessler: BioSight Ltd.: Current Employment. Blumberg: BioSight Ltd.: Current Employment. Gengrinovitch: BioSight Ltd.: Current holder of individual stocks in a privately-held company. Ben Yakar: BioSight Ltd.: Current Employment. Rowe: Biosight Inc.: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal